Mukthinuthalapati Mathrusri Annapurna* and Vejandla Swathi Lakshmi
Department of Pharmaceutical Analysis, GITAM School of Pharmacy, GITAM (Deemed to be University), Visakhapatnam, India
*Corresponding Author: Mukthinuthalapati Mathrusri Annapurna, Department of Pharmaceutical Analysis, GITAM School of Pharmacy, GITAM (Deemed to be University), Visakhapatnam, India.
Received: October 14, 2024; Published: October 27, 2024
Citation: Mukthinuthalapati Mathrusri Annapurna and Vejandla Swathi Lakshmi. “Analytical Methods for the Assay of Tucatinib – A Review". Acta Scientific Pharmaceutical Sciences 8.11 (2024):37-38.
Tucatinib is anti-cancer agent. Tucatinib acts by binding to the human epidermal growth factor receptor 2 (HER2) protein and thereby preventing it from sending the signals that promote the cell growth. It is a tyrosine kinase inhibitor. In the present study the authors have reviewed and summarised the analytical methods so far developed for the estimation of Tucatinib in pharmaceutical formulations as well as biological fluids.
Keywords: Tucatinib; RP-HPLC
Tucatinib (CAS: 937263-43-9) (Mol wt: 480.5g/mol) is a tyrosine kinase inhibitor showing anti-tumour activity. It is soluble in organic solvents such as DMSO and dimethyl formamide and sparingly soluble in aqueous buffers. Tucatinib is used in combination [1-3] to treat breast cancer, unresectable breast cancer, wild type Ras metastatic colorectal cancer, unresectable Ras wild type colorectal cancer.
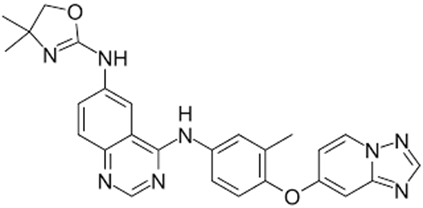
Figure 1: Chemical structure of Tucatinib ((C26H24N8O2).
Tucatinib is available as tablets with brand names Tucaxen 150 (Everest) Tukysa (Seetle Genetics), TukadxTM (Bigbear Pharmaceutical Laos) etc and label claim 150 mg. The analytical methods such as LC-MS/MS [4], UPLC-MS/MS [5], RP-HPLC [6,7] methods so far developed for the estimation of Tucatinib were given in Table 1.
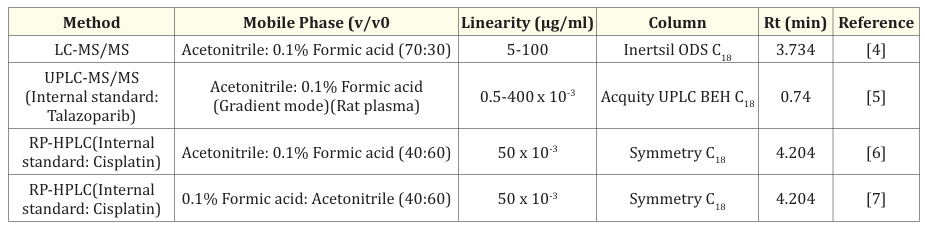
Table 1: Review of Analytical methods.
The authors have reviewed the analytical methods for the estimation of Tucatinib in pharmaceutical dosage forms as well as biological fluids.
Copyright: © 2024 Mukthinuthalapati Mathrusri Annapurna and Vejandla Swathi Lakshmi. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.