KT Parthiban*, MV Jawahar Vishnu, S Revathi, T Bhuvaneshwari, R Hindumathi, P Kumar, Deepshikha Singh and B Santhoshkumar
Forest College and Research Institute, Tamil Nadu Agricultural University, Mettupalayam, Tamil Nadu, India
*Corresponding Author: KT Parthiban, Forest College and Research Institute, Tamil Nadu Agricultural University, Mettupalayam, Tamil Nadu, India.
Received: September 23, 2024; Published: October 03, 2024
Citation: Sandeep Kumar., et al. “Biofortification in Field Crops: A Cost-Effective and Sustainable Breeding Approach for Demoting Hidden Hunger". Acta Scientific Agriculture 8.10 (2024): 01-03.
Mass multiplication of true-to-type planting stock with all the genetic superiority of the parent material is possible through the application of clonal propagation techniques. The development of miniclonal technology allowed the concept of intensive management of producing vegetative propagules to be achieved at commercial scale as for cuttings systems for large scale production of vegetative propagules. Investigations were carried out for standardizing Miniclonal Technology for economically significant multiutility industrial tree species viz., Melia dubia, Khaya senegalensis, Toona ciliata, Chukrasia tabularis, and Acacia hybrid. The mini cuttings were exposed to various rooting hormones specifically IBA at concentrations of 1000, 2000, 3000, 4000, 5000, and 6000 ppm respectively. Results indicated that the rooting percentage recorded was found to be higher in Acacia hybrid (79.16 % @1000 ppm) followed by Melia (77.50 % @ 2000 ppm), Toona ciliata (65.00 % @ 2000 ppm), Khaya senegalensis (62.50% @ 3000 ppm) and Chukrasia tabularis (51.66 % @ 1000 ppm).
Keywords: MCT; Industrial Wood Species; QPM
The expansion of Trees Outside Forests is gaining significant attention and attraction among the stakeholders to produce the wood and non-wood forest products amenable for domestic and industrial utility. The nation has documented 28.42 million hectares dedicated to agroforestry with a variety of species [1]. Even though trees in non-forest areas have always been important in India's traditional land use, their significance for fulfilling industrial raw material requirements has only emerged recently. The wood-based industries have grown both horizontally and vertically over time, in terms of installed capacity and the associated product out-turn. This growth, along with higher product demand, has led to a significant increase in the wood demand. Accordingly, for the State of Tamil Nadu the wood demand for major sector of industries were estimated to be > 33 lakh tonnes (timber), 12.16 lakh tonnes (ply and panel), > 17.60 lakh tonnes (pulp and paper), 2.67 lakh tonnes (packing case), > 4.50 lakh tonnes (matchwood), 97.83 lakh tonnes (energy) and > one lakh tonnes (miscellaneous industries) [2]. The growth of agroforestry and the need for raw materials in both domestic and industrial sectors have not kept pace with each other because of the many challenges and limitations present in the entire Production to Consumption System (PCS). One of the key challenges is ensuring the availability of Quality Planting material (QPM) derived from genetically improved resources, which is crucial for both productivity and sustainability [3]. The country has placed a high importance on promoting agroforestry through the use of Quality Planting Material (QPM), but progress in this area has unfortunately been limited, apart from a few industrial trials. In order to meet the demand for Quality Planting Material (QPM) as quickly as possible, clonal forestry has emerged as a novel and innovative technology.
Initially, most plantation activities involved producing seedlings from seeds, which exhibited a diverse range of productivity and adaptability. In spite of this, commercial planting began in the 20th century, and in the 1980s, the first clonal plantations for poplar and eucalyptus were established [4]. The main objective of vegetative propagation in general, and clonal propagation in particular, is to create plantation of identical trees with excellent wood quality and high-volume growth. On the other hand, the commonly utilized method of rooted stem cutting has multiple disadvantages which include a substandard root system which negatively impacts the genetic expression of numerous clones, rapid decline in rooting ability due to ontogenetic aging, and variation within clones caused by topophysis. This issue has been resolved by the use of a novel technique known as mini clonal technology, in which nutrition management and a controlled environment are used to develop the clonal mother plants which are further utilized for mass multiplication [4]. The technique is seen as a cost-effective and efficient way for quicker and simpler multiplication, with a strong chance of replacing rooted stem cuttings due to its technical and economic benefits, along with the demonstrated success of rooting in auxiliary shoots.
Propagation through mini-cutting is considered the most advanced commercial cloning method in India. Regular cuttings are usually taken from shoots grown in the field after being coppiced and are characterized by a stem section that is more lignified and has two trimmed leaves. Nevertheless, the new generation of mini cuttings enhances the quality of cuttings from well-maintained planting stock and speeds up clonal multiplication, thus shortening the breeding and selection cycle. Mini clonal technology is characterized by the use of young plants as the source of vegetative propagules. Shoot tips are used as mini-cuttings, which are subsequently placed in an appropriate rooting medium and placed in a greenhouse with regulated humidity and temperature to initiate the rooting process. The shoot apex plays a crucial role in maintaining the root system's quality as it leads to the development of a taproot-like system [5]. In this context, Forest College and Research Institute in Mettupalayam has initiated the study on standardizing Miniclonal Technology for economically significant multiutility industrial tree species such as Melia dubia, Khaya senegalensis, Toona ciliata, Chukrasia tabularis, and Acacia hybrid.
Miniclonal technology in General is a common practice in species like Eucalyptus and Casuarina. However, such studies have not been carried out in tropical species. The current investigation was carried out to study the feasibility of mini-clonal propagation in five tropical tree species viz., Melia dubia, Khaya senegalensis, Toona ciliata, Chukrasia tabularis, and Acacia hybrid.
The study was conducted at Forest college and research Institute, Mettupalayam, India, located at 11 ̊ 9’N latitude and 77 ̊ 56’E longitude a region characterized by its subtropical climate and elevation of 300 meters above MSL. The experimental material consists of five tree species, selected based on their prolific growth, superior morphogenic characteristics and multi-industrial utility.
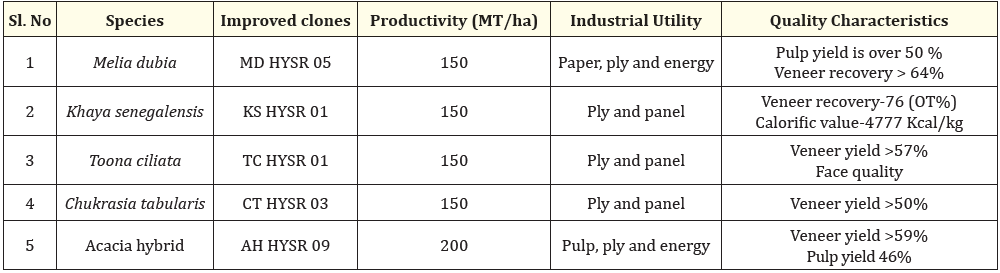
Table 1: List of multiutility industrial tree species used for the study.
Selecting the most promising candidate plus tree (CPTs) for clonal propagation is the first step in this process. This method involves selecting specific trees with superior morphogenic characteristics from the existing plantations (Seed source/progeny evaluation Trials). Trees are chosen based on their growth characteristics, physical appearance, overall health, apical dominance, productivity and wood quality. Typically, superior trees are chosen from established plantations using a comparison or check tree method. Selection is also influenced by the species, management goals, end use, and commercial value. After selecting the trees, they are cut at the bottom, typically 15 cm above the ground level, and then left to coppice. These young shoots from coppiced trees are collected are treated with a rooting hormone and ramets are multiplied and then planted in the clonal mother gardens for further mass multiplication (or) the young shoots (apical shoots) from coppiced trees are collected and propagated through tissue culture under in vitro conditions. Once required number of plants are multiplied, these plants are hardened and established in the clonal mother gardens.
The mini cuttings were collected from the CPTs using sterilized secateur most probably in the morning hours. The mini cuttings were sorted based on the desired length (7-12 cm) and diameter (3-5 mm). The mini cuttings were treated with aqueous solution of 0.1% Bavistin (Carbendazim 50% WP, Systemic fungicide) for 15 minutes to prevent wide range of pathogenic fungal diseases. Subsequently, the mini cuttings were exposed to various rooting hormones specifically IBA at concentrations of 1000, 2000, 3000, 4000, 5000, and 6000 ppm respectively. The treated cuttings were placed in a rooting mixture of coir pith and perlite (70:30) in root trainers. Coir pith has good water retention and sustainability, while perlite has great drainage and aeration. Later on, the cuttings were placed in a mist chamber where the cuttings were periodically misted (60 seconds for every 30 minutes) to maintain the humidity at 60-80% and the temperature at 25-30°C during the day and 15-20°C at night. After a period of 30 days, the cuttings were taken out of the rooting medium and data regarding rooting percent, root length, shoot length and collar diameter were recorded and analysed in SPSS.
Coppice cuttings from 1-2-year-old plants were used in traditional clonal technology, which is laborious, time-consuming, and also the ability of the rooting decreases with ontogenetic ageing [6]. The earlier studies also suggests that the ability of rooting reaches maximum at high juvenility level [7]. The results in Table 2 reveals variations in recorded parameters primarily in rooting percent which is attributed to plants own ability to differentiate and dedifferentiate [8,9]. The results indicate that the rooting percentage recorded was significantly higher in Acacia hybrid (79.16% @1000 ppm) followed by Melia (77.50% @ 2000 ppm), Toona ciliata (65.00% @ 2000 ppm), Khaya senegalensis (62.50% @ 3000 ppm) and Chukrasia tabularis (51.66 % @ 1000 ppm). The ability of the plants to form roots is a heritable quantitative genetic trait controlled by internal and external factors mainly carbohydrate reserves, auxin, and light conditions [10]. The results are in line with previous studies [11-14] on similar species at different IBA concentrations. The results show that the application of rooting hormones, specifically auxin in the form of IBA on mini-cuttings, significantly aided in the rooting of the different tropical forest tree species considered under the study. This is explained by the way auxin affects the hydrolysis of polysaccharides, which produces physiological active sugar in the cells and encourages the meristematic tissues to create adventitious roots. Numerous researchers have previously reported that IBA is superior to other hormones in Acacia albida [15], Woodfordia floribunda [16], Parkia biglandulosa [17], Ceiba pentandra [18] and Pterocarpus dalbergioides [19] and Lannea coromendalica [20].
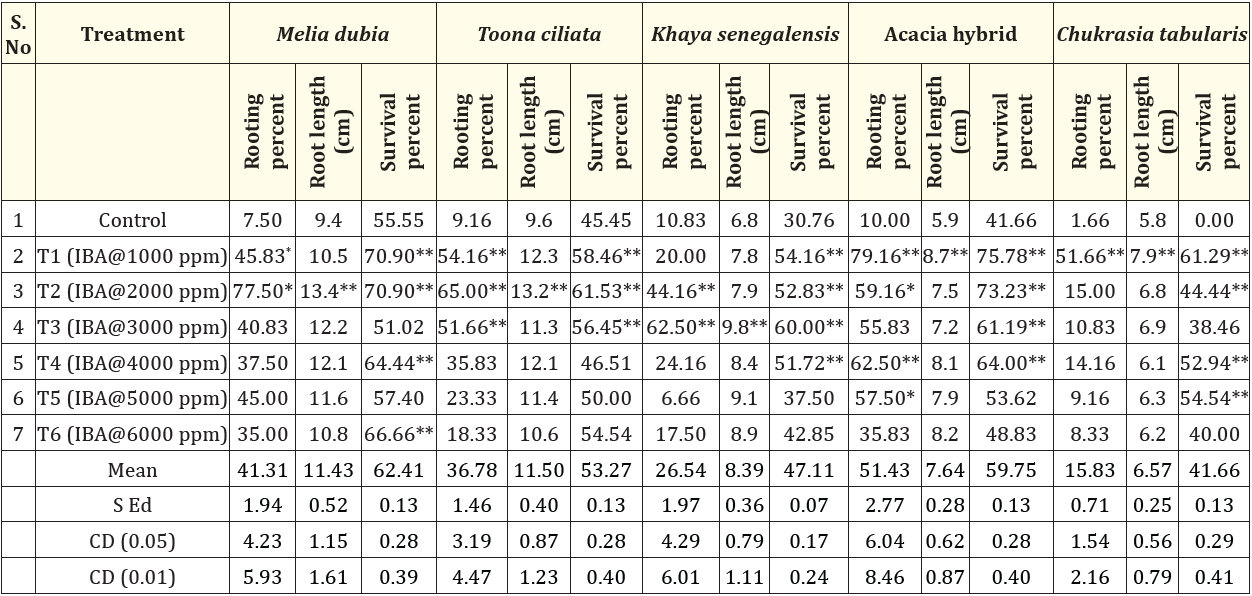
Table 2: Mini cutting studies of selected multiutility industrial tree species.
The root length exhibited significant variations among the treatments. The root length varied from 13.40 cm (Melia dubia) to 7.90 cm (Chukrasia tabularis). This might be associated with auxin action, which may have been triggered by the transfer and hydrolysis of carbohydrates to the cuttings base, causing cell elongation and division. Numerous species have been reported to benefit from exogenous hormone treatment, particularly growth regulators like IBA, which are known to encourage root length. The results are in agreement with the earlier findings [5,21-24].
In the current study, among the several hormonal concentrations, the maximum survival percentage was observed in in Acacia hybrid (75.78% @1000 ppm) followed by Melia (70.90% @ 2000 ppm), Toona ciliata (61.53% @ 2000 ppm), Chukrasia tabularis (61.29% @ 1000 ppm) and Khaya senegalensis (60.00% @ 3000 ppm).The overall recorded results in the present nursery experiment revealed that different cutting length/diameter and IBA application has positive effect on the morphological characteristics of tropical forest tree species [25]. The lower survival percentage may be attributed to the degree of lignification in the primary phloem of mature, woody cuttings affects the rooting ability of cuttings by hindering the root primordia tissue from developing root initials.
The increase in wood demand coupled with requirement of Quality Planting Material with improved wood quality and productivity have necessitated the development of miniclonal technology for tropical tree species. Presently, no standardized protocols are available for clonal propagation of tropical tree species using apical shoot cuttings. The current study indicated that IBA@1000 ppm registered the highest values for important root traits in Acacia hybrid and Chukrasia tabularis, IBA@2000 ppm registered highest values for Melia dubia and Toona ciliata and IBA@3000 ppm registered highest values for Khaya senegalensis. The present investigation has standardized the rooting hormone concentration for the tropical tree species which is found to be a promising method for mass multiplication of Quality Planting Material in the shortest possible time with a high success rate that has the potential to replace the conventional seed propagation. Mini clonal technology ensures uniformity and maximizes the quantity and quality of production for forest-based industries when compared to seed-based plantations.
The authors profusely thank the Department of Biotechnology (DBT), Government of India, New Delhi for supporting the research scheme on development of HYSR clones amenable for multifarious industrial utility (BT/PR/39385/FCB/125/42/2020) in which the current study formed a part of the programme.
Copyright: © 2024 KT Parthiban., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.